Пристрій для витягнення золота з промислових відходів
Формула / Реферат
1. Устройство для извлечения золота из промышленных отходов, включающее герметичную камеру для выщелачивания, в корпусе которой выполнены отверстия для ввода исходного сырья, ввода выщелачивающего раствора и отвода золотосодержащего раствора, снабженную перемешивающим устройством, камеру для сбора золотосодержащего раствора и фильтр, отличающееся тем, что в корпусе камеры для выщелачивания дополнительно выполнено отверстие для отвода образующих газов, а фильтр расположен внутри камеры для выщелачивания и представляет собой фильтровальный материал, натянутый по крайней мере на одну поверхность пластины, в которой выполнены концентричне расположенные углубления и радиально расположенные углубления, в пластине также выполнено отверстие, расположенное на краю пластины и не имеющее выхода на противоположную сторону пластины, образующее в пластине полость, а также канал, который соединяет полость пластины с одним из радиальных углублений, причем отверстие, не имеющее выхода на противоположную сторону пластины, соединено с отверстием в корпусе камеры для выщелачивания для отвода золотосодержащего раствора.
2. Устройство по п.1, отличающееся тем, что при выполнении углублений с двух сторон пластины и натяжении фильтровального материала на обе ее поверхности в пластине дополнительно выполнены центральное отверстие и прорезь, соединяющие между собой радиальные углубления, расположенные по обе стороны пластины.
3. Устройство по п.1, отличающееся тем, что образующая боковой поверхности пластины соответствует образующей внутренней боковой поверхности камеры для выщелачивания, а расстояние от края пластины до внутренней боковой поверхности камеры для выщелачивания составляет 2,5 - 3,0мм.
4. Устройство по п.1, отличающееся тем, что расстояние от нижней поверхности фильтра до днища камеры для выщелачивания составляет 5,0 - 10,0м.
Текст
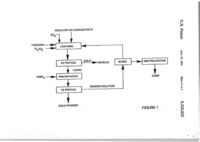
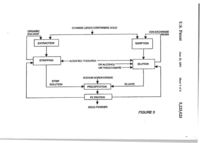
US005223023A United States Patent m Awadalla et al. [11] Patent КшпЬег: [45] Date of Patent: [54] 5,223,023 Jim. 29, 1993 4,992,200 2/1991 Lin et al RECOVERY OF GOLD FROM SOLUTION BY REDUCnON.PREClPITATION WITH STABILIZED ALKALI METAL BOROHYDRIDE [75] FOREIGN PATENT DOCUMENTS 1197986 12/1985 Canada 158B28 12/1981 Japan Inventors: Farouk T. Awadalla, Hull; Gordon M. Ritcey, Nepean, both of Canada [73] Assignee: Her Majesty the Queen is right of Canada, as represented by the Mmister of Energy, Mines and Resources, Canada 423/24 75/741 75/741 OTHER PUBLICATIONS 55-158828 Japan Abstracts Dec. 7, 1981. Primary Examiner—Melvyn J. Andrews Attorney, Agent, or Firm—Sughrue, Mion, Zinn, Mac[»eak & Seas [21] Appl. No.: 649,388 [57] [221 Piied^ A process is disclosed for the direct recovery of gold Ггош thiourea, thiocyanate or thiosulfate solutions or acidified thiourea leach liquor. The process comprises reduction precipitation of Oie gold from solution by the addition of stabilized alkali metal borohydride, prefera bly sodium or potassium borohydride, at ambient tem> perature and pressure, The resulting gold precipitate is generally of high purity and is readily separated fay filtration. The barren solution is in a condition such that it can be recycled to the upstream process. [30] Jan. 30,1991 Foreign AppUcation Priority Data Mar. 30, 1990 tCA] Canada 2013537 [51] Int. а . ї [52] U.S. a [58] Field of Search [56] C22B 3/22 75/741 75/741; 423/24 References Qted U.S. PATENT DOCUMENTS 4,096.316 6/1978 Tamai et al 4,913,730 4/1990 Deschenes el al 75/370 75/370 ABSTRACT 24 Claims, 4 Drawiag Sheets GOLD ORE OR CONCE^fmATE SO, THIOUREA ^ H ЧП. — ^ ^ IJ LEACHING ^ V FILTRATION 1f NaBH^ ^ ,-€fQj^^ ^^"-'^te nC-IDUE і' UQUID ^ - PHEClPrTAtlON і RLTRATION і GOLD POWDER BIFFD — ^ NELfTRAUZATlON і f SUMP BARREN SOLimON « GOLD ORE OR CONCeiTRATE SO. THIOUREA H2SO4 ll I ГО LEACHING С-1 с 9 № О FILTRATION NaBH, I Ї Ы | _ S O L ' P ^ дЕзірцЕ UQUID I NEUTRALIZATION Ї ^ BARREN SOLUTION 2. FILTRATION ©OLD POWDER ы SUMP PRECIPITATION I BLEED СП пбшті о « ' OOLD ORE OR CONCENTRATE SO I ; THIOUREAHgSQi LEACHING IN PRESENCE OF CARBON CIL і SOUD RESIDUE AND UQUID TO FURTHER TREATMENT OR WASTE SIEVE RLTRATION SODIUM THIOSULFATH OR SODIUM THIOCYANATE NaBH, FJ SOUD CARBON EUmON і ELUATE (LIQUID) PRECIPITATION ? FILTRATION T GOLD POWDER BARREN SOLUTION FIGURE2 CYANIDE LEACH CONTAINING GOLD і ORGANIC SOLVENT L^ 1£ _ EXTRACTION STRIPPING ION-EXCHANGE RESIN SORPTION -ACIDIFIED THIOUREA ELUTION OR ALCOHOL OR THIOCYANATE SODIUM BOROHYDRIDE STRIP SOLUTION I PRECIPITATION ELUATE FILTRATION —Ї GOLD POWDER FIGURES GOLD CYANIDE LEACH -NaOH SOLmiON АОЮ NaCN FOR CYANIDATION PROCESS Au(l)-THIOUREA с on NaBH4 ^ g n Au(l) - ACID SOLUTION Ы ]L_i VO PRECIPITATION I FILTRATION tP fD UQUtDFCpy(M0TE О 4k t GOLD POWDER FIGURE 4 о 5.223,023 1 2 Hydrogen reduction is another method that has been RECOVERY OF GOLD FROM SOLUTION BY used to recover gold from thiourea solution This proREDUCnON-PRECTPITATION WITH cess requires high temperatures and pressures and the STABILIZED ALKALI METAL BOROHYDRIDE use of a catalyst which contributes to high operatmg S costs. As well, the reaction kinetics are quite slow. BACKGROUND OF THE INVENTION Electrolysis has also been used in the recovery of ,. J gold from thiourea solution on a commercial basis. This L Field ot the invention method requires an elaborate two-stage electrolysis This invention relate to a process for the recove у of with special cell design in order To obtain suffi. metric goldfromgold-comammg thiourea thiosulfate ,^ ,І,„Й щ ь recoveries. or tbocyanatc solutions. ТЪе mvention further rela es to a process for the recovery of metallic gold generally of bgh punty from pregnant solutions as a r«uh of the leachmg of gold ore or concentrates by acidified thio'^^^^««^^ж-^.лжт-ч^-г-тт-ж,^. іт-г-т-. іп-г т^с reducmg power of sodium borohydride has long ^ ^ „ploited foVmdustrial appUcations such as poUu^.^^ ^^^^^^^ ^^ ^^^ ^^^^^^ ^ ^ ^ ^ ^ ^ ^ ^ ^^ ^ ^ _ ^ ^ ^^^j ^^^^^^ ^^^^ ^^^.^^ Currently, sodium bor15 ohydnde IS fmdmg apphcation m the recovery of silver 2. DESCRIPTION OF THE RELATED ART ^J^ photcgrapHc hquor (thiosulfate solution), as Hitherto, there have existed two рппсфаі methods of ^^^^^^^ ^ ^ g p^^ ^ ^ 3.082,079. or spent electrolyte rcco^nng gold from gold ores or gold concentracs. ^ ^ ^^^^^ ^^^^ ^^^^ ^^.^.^ j ^ ^ j ^ ^ ТЪе first method mvolv«cyamdation followed by the ^^^^^ ^^^^ ^^^ ^^^^^ ^^^^ ^ (.^2+. Fe3+/2+, Merill-Crowe process wherein gold IS recovered from jo N,2+ , Hg2+. Co^+and Pb^+can be removed from toxic solution by cementation with zmc powder which must ^^^^^^ ^ ^^^ borohydride treatment. However, then be refined to obtain gold metal The process offers ^^еге has been no suggesUon of a sodium borohydride high gold recovery, but with low punty. The second reduction process for the recovery of gold from leach method comprises cyamdation followed by recovery ^^^^^^^ ^^^^^ j ^ ^^ ^ (Canadian Patent No. 1,090,584) using activated carbon followed by electrolysis. The 35 teach a reduction precipitating agent containing alumicarbon-in-pulp (GIF) process mvolves contact belwecn ^^^_ ^ ^jj.^, ^^^^ borohydride and a hydrazme comthe activated carbon and leached pulp. Absorption of ^^^^^ f^^ recovering precious metal values includmg impurities onto the carbon and the difficulties of desorpg^j^ ^^^^ aqueous alkaline cyanide solutions This prior «on of the gold are distinct disadvanuges of this prop^^^^^ ^^^^^ ^^^^ cyanide effluent problems as well cess. The carbon-m-leach (CIL) process mvolves load- 3^ ^ material losses due to the necessity for cyanide efiluing the gold onto the carbon dunng leachmg. In both ^^^ destruction, the CIL and GIF processes, the precious metal must be eluted and passed to an electrowinning step for gold SUMMARY OF THE INVENTION recovery Gold recovered on the cathode then requires j ^ j^ j ^ object of this mvention to provide a simple further refining The activated carbon can be regener- 35 and economic method for recovering high purity metalated and then recycled. Ijc goij directly from aqueous solutions includmg thioEach of the these prior methods mvolves cyanidaцгеа, thiosulfate and thiocyanate solutions, tion. Because of the toxicity of cyanide, additional steps ц jg 3 further object of this mvention to provide a arc required for its handling and subsequent ehmination method for the recovery of metallic gold from thiourea This significantly increases the operating costs of these 40 і^сЬ hquor resulting from the leaching of gold ore or processes concentrate by acidified thiourea. A recently proposed less toxic alternative to cyanide Accorduigly, the invention provides a process for for leachmg gold ore or gold concentrate is thiourea recovering high purity metallic gold from an acidic However, thus far. there is no well estabUshed method solution containing gold values, which comprises: addof recovering gold from non-toxic reagents such as 45 ing ю the solution stabihzed alkah metal borohydnde, thiourea solution. Attempts have been made to recover preferably sodium or potassium borohydride, in an gold by thiourea leaching followed by precious metal amount at least stoichiometricaljy equal to the amount recovery from solution by aluminum cementation, actiof gold compounds present ш solution, separating the vated carbon, ion exchange, solvent extraction and resultant metallic precipitate by filtration; and, optionelectrolysis. However, these prior methods all have one 50 ally, recycling the barren solution to the upstream proor more disadvantages. As well, there is often thiourea cess. decomposition durmg gold recovery from the pregnant in a preferred embodiment of invention, reduction solution which adds cost by decreasuig the amount of precipitation is effected by stabilized sodium borohybarren thiourea that can be recycled dnde ш the form of Ven Met TM solution (12% NaBH*, Thiourea IS currently an effective eluate for resin 55 40% NaOH). loaded with gold cyanide complex and as a stripping In a further preferred embodunent, reduction precipiagcnt for gold from organic solvent loaded with gold tation is effected by stabilized potassium borohydride from cyanide media However, no satisfactory method ^ _ _,„ has been developed to recover gold from such solui S ^ ^ ^ * ^ ' ^ °^ ™^ One method of recovering gold from such thiourea Extensive study has revealed that an important factor solutions involves neutralizmg the acidified tbourea ш the use of stabilized sodium borohydride for reducsolution to a pH of about 6.5 which results ш the prcciption precipitauon is pH control. Normally, borohydride iiation of gold due to pH change. However, the method reduction is effected at a pH of from 5 0 to 7 0. Below a IS non-sclective and uneconomical due to the acid con- 65 pH of 5.0 borohydride tends to hydrolyze wbch results sumption necessary to readjust the solution pH if the man increased consumption ofthe borohydride. On the thiourea is to be recycled. As well, thiourea is relatively other hand, thiourea is stable in acidic medium, but at unstable at a pH above 4. higher pH levels undergoes degradation. Therefore, it З 5,223,1 4 was unexpected when it was found that sodium borohy TABLE 1 dride reduction in an acidic solution of thiourea was Reduction of gold ion by SBH in ihJoure* folution efficient. It appears that the alkaline content of Ven Met contunug difTcrent concentrat[oiu of gold TM solution acts to stabilize sodium borohydride m an (CondtiioiU: V = 100 mL, itoichiometry (mole SBH/mole Au) acidic pH range and substantially prevents its hydroly- ' 9.4, thiourea =Б 7 g/L, initial pH эс I.S, rime — 2 шіп) sis. Through extensive experimentation it was found pH, Au recovery. Au+concn. Au b ulution that the reduction efficiency of stabilized sodium boro (inti mg/L mg/L % hydride peaked at approximately pH 2.5. Furthermore, 2.58 0.06 97.0 1 it was found that more than satisfactory reduction effi Ч7.0 2.73 0.16 S ciency occurred when using stabilized sodium borohy 0.0 100.0 3.IE 10 dride in the pH range of about 1.5 to 3.0. 10.29 0,0 100.0 20 100.0 50 9.12" 00 Analysis has shown that the concentration of thio 99.95 2.23»* 0.05 100 urea in the pH range of 1.0 to 3.0 is stable before and after the reduction reaction. It is the stability of thiourea jj *Ini;Ul pH — 1.0. al pH > 3.0 (otd *un to pncipiute "lutUt pN « 1.3 Ьеошсаі pH > 1.5, ргапріипопоГ|ои iianed during the reaction that allows it to be in recyclable condition which contributes significantly to reducing Accordingly, reduction precipitation using sodium the operating cost of the process. borohydride is effective in thiourea solutions substan For gold-containing thiosulfate solution, peak effi tially independent of gold concentration. Barren thio ciency for reduction precipitation is at about pH 6.0. In 20 urea solution, after the separation of precipitated metal, the pH range below 4.0, a yellowish-white precipitate was found to contain less than 0.2 mg Au/L. forms. In untreated thiourea leach liquors, precious metals For gold-containing thiocyanate solution, maximum contained therein are usually associated with foreign reduction efficiency occurs at about pH 2.5. contaminating ions such as copper, iron, cobalt, nickel For gold-containing thiourea solution, reaction by 25 and zinc. Therefore, it is necessary to know their effect the addition of stabilized sodium borohydride in the on the reduction of gold using sodium borohydride. form of Ven Met TM solution was found to reach maxi It has been found by experimentation that the pres mum efficiency after thirty seconds. For gold-contain ence of zinc ions enhances the reduction efficiency of ing thiosulfate solution, maximum efficiency of the re duction reaction was found to occur after 1 hour at pH 30 gold. For example, the presence of 10 mg/L zinc ion in thiourea solution containing 10 mg/L gold was found to 6.0. For gold-containing thiocyanate solution, maxi decrease sodium borohydride consumption by B3% mum efficiency was found to occur after about 30 min independent of pH wittUn the operable pH range for utes. thiourea. Furthermore, the gold precipitate was ana The reduction precipitation cf gold in gold-containlyzed to be zinc free. It appeare that sodium borohy mg thiourea solution proceeds according to the follow dride is inefficient at reducmg zinc ion. ing reaction: The presence of other metal ions such as iron (II), cobalt, nickel and copper were found to produce a 8Au[CS(NH:)2li+ + BH4-—BAu" negative affect on gold reduction by sodium borohy 4-16CS{NH2):+B02-+8H-^ 0) 40 dride. The amount of decrease in reduction efficiency varied with each ion in the following order; F e > C o = It can be clearly seen in equation (I) that eight elec Ni>Cu. The precipitated product obtained in the pres trons per molecule of sodium borohydride arc available ence of these ions was analyzed to be substantially pure to reduce gold ions. When complexed with acidic thio gold indicating that although their presence hinders the urea, gold is present in monovalent form. Thus, accord ing to the stoichiometry of the reaction, eight moles of ^^ reduction efficiency, they are not reduced to any signifi cant degree. That is, they do not contaminate the final gold are reduced for each mole of sodium borohydride. product. Therefore, the inventive process is still opera In practice, however, an excess amount of NaBHi is ble for gold-containing solutions which also contain the required as a result of the necKsity for a low pH. It has contaminating ions Fe, Co, Ni and Cu. been found that the stoichiometric amount of sodium borohydride increases as pH decreases. For example, It has further been found that the presence of silver for pH values of 1.5,2.0.2.5 and 3.0, molar ratios of 100, enhances the reduction efficiency of gold with sodium 25, 10 and 3, respectively, of КаВЩ/Аи were required borohydride in thiourea solution. Moreover, the reduc to effect reduction. For thiosulfate and thiocyanate tion efficiency increases as the concentration of silver gold-containing solutions, the stoichiometry is even less j j increases. For example, it was found that in the pres than unity as a result of the pH of reaction being nearly ence of 10 mg/L and 100 mg/L of silver ion, the neutral (pH-6.0). amount of КаВНд consumed decreased by 33% and 83%, respectively, over the amount consumed when no It has further been found that the concentration of silver was present. Therefore, in cases where gold and gold has little effect on the efficiency of the NaBH* reduction reaction. As can clearly be seen in Table 1, go silver are in a mixture, reduction can be performed in two stages to precipitate gold and silver metal sepa the percentage recovery of gold remains substantially rately in good yield and high purity. It can be seen in constant over a large range of gold concentration (from Table 2 that the reduction of gold in thiourea in the 2 to 100 mg Au/L solution). This is a significant advanpresence of silver, copper and iron (11) in only one stage Uge of the invention which allows application of the process to solutions of thiourea as both a lixiviant 65 resulted in a product of medium purity. However, as shown in Table 3, if reduction is effected in two stages, (wherein the resultant leach liquor has rather small gold gold and silver precipitate separately in good yield and concentrations) and a stripping solution (wherein gold high purity. concentrations are high). 5,223,023 5 TABLE 2 Rcductian of mixture with SBH in one stage ComposilioD of solution, mg/L ~ Au — 50, Ag • SO, Cu= 25 ajid Fe = 25, Tu = 7 g/L, V ss 100 cc, time = 5 min, T = 25' C . pH initial = 1.66. SBH/Au = 12.5 liquor which incorjmrates a recovery step as embodied by the mvention. DESCRIPTION OF THE PREFERRED EMBODIMENTS With reference to FIG. 1, in a preferred embodiment of invention gold ore or concentrate is leached by acidi fied thiourea. Any solid residue remaining in solution is 9B.74 S7.0 0.63 Au separated by filtration and the resultant liquid is treated 17.09 65.B3 31.0 A| 21.5 14.00 Ca 4.0 10 with stabilized sodium borohydride to effect reduction 24.22 Э.12 Fe l.O precipitation. Precipitated gold powder is recovered by filtration and the barren solution is optionally (upon treatment for impurity control) recycled to the up TABLE 3 stream leaching stage. KeductioB of mixture with SBH in two stages 15 In the embodiment shown in FIG. 2, gold ore or Initial pH » 1.5 and 2.68 for 1st and 2nd stage concentrate is leached with acidified thiourea in the 1st lUge SBH/Au = 9.375 presence of carbon (conventional CIL method). Carbon 2nd stage SBH/Au = 3-125 is separated by sieve filtration and the remaining ore Concn. of metal residue and liquid is further treated or expelled as waste. Recovery, ion in banco Purity, solution mg/L 20 The separated carbon is eluted with sodium thiosulfate % 1st 2nd Metal Ul 2nd 1st 2nd or sodium thiocyanate and the resultant eluate is then stage Stage SUge stage stage SUge ion separated and treated with stabilized sodium borohy 1.24 89.30 76,S5 dride to induce reduction precipitation. The precipi Au 5.348 83,2 10.9 «.30 13.8S 9,60 82.8 45.2 В.Э Ag tated gold powder is recovered by filtration. Option 7.60 0.0 21.19 21.48 7.1 0.0 Ot 25 ally, the barren solution can be recycled to the upstream 0,84 9.7 24.5S 22.19 0.8 6.3 Fe (stripping) elution stage. With reference to FIG. 3, in further embodiments of In the temperature range of 25*to 80" G. reduction invention gold-containing cyanide leach liquor is efficiency was found to be substantially stable. A treated by either of two methods: slightiy increased efficiency was obtained at higher 30 1) The leach liquor is treated with an organic solvent temperatures. However, it was found that the increased to extract gold therefrom. The loaded organic phase is efficiency achieved was more than offset by the in stripped with acidified thiourea solution. The gold-con creased cost associated with the higher reaction temper taining thiourea strip solution is treated with stabilized ature. Accordingly, ambient temperature is the pre sodium borohydride to induce reduction precipitation 35 of gold. The precipitated gold metal powder is recov ferred temperature to effect reaction. ered by filtration. Optionally, the strip solution is recy The recovery of gold from solutions using stabilized cled to the upstream stripping stage. sodium borohydride in the form of Ven Met TM solu 2) The gold-containing cyanide leach liquor is treated tion is efficient, fast, simple and low in cost. In terms of with an appropriate resin resultmg in the sorption of cost it is estimated that at present prices one pound of high purity gold can be recovered through the use of 40 gold thereon. The loaded ion-exchange resin is sepa rated and eluted with acidified thiourea, alcohol-con SIO worth of Ven MetTM solution, based on a per taining solution or thiocyanate. Gold powder precipi pound Ven MetTM solution price of $2.3 to $3.3. tate is formed as a resuh of the treatment of the eluate A key advantage of the invention is that recovery of with stabilized sodium borohydride to induce reduction high purity gold by reduction precipitation with a stabi 45 precipitation. Gold powder is recovered by filtration lized alkali metaJ borohydride can be employed in the and the barren eluate is optionally recycled to the up fmal steps of already established and commercially via stream eluting stage. ble gold recovery processes as will be demonstrated in In the embodiment of invention shown in FIG. 4, the following paragraphs. gold-containing cyanide leach liquor is treated with BRIEF DESCRIPTION OF THE DRAWINGS 50 acid or acidified thiourea. Liberated hydrogen cyanide gas is recovered by treatment with sodium hydroxide Further objects, features and advantages of the inven solution to form sodium cyanide which can be recycled tion will become apparent to those skilled in the art for use in the cyanidation of gold. The liquid stream from the following description thereof when taken in from the acidification process (either Au (I) - thiourea conjunction with the accompanying drawings, in 55 or Au (I) - acid solution) is treated with stabilized so which: dium borohydride to effect reduction precipitation. FIG. 1 shows a process flowsheet for the leaching of Precipitated gold powder is recovered by filtration and gold ore or concentrate by acidified thiourea which the filtrate is expelled as waste. includes a recovery step as emtxxlied by the invention; The following examples further illustrate the mven FIG. 2 shows a process flowsheet for the leaching of щ tion: gold ore or concentrate with acidified thiourea in the EXAMPLE 1 presence of carbon including a recovery step as embod ied by the invention; A 100 ml test solution containing 100 mg/L Au and FIG. 3 shows a process flowsheet for the treatment of 7g/L thiourea solution at pH 1.5 was stirred gentiy by gold-containing cyanide leach liquor for the purpose of 65 a magnetic bar. A calculated amount of stabilized so recoving gold as embodied by the invention; and dium borohydride in the form of Ven Met TM solution FIG. 4 shows a process flowsheet for an additional was added to the test solution which immediately re method of treatment of gold-containing cyanide leach sulted in the formation of a fine black precipitate. The Metal ion Сопел, of barren solution, mg/L Punty of product. Recovery, % % 5.223,023 product was separated on fiher paper and analyzed by x-ray diffraction. It was revealed to be substantially pure metallic gold. Scanning electron microscope anal ysis also revealed that the particles were very fine with 1 diameter of less than 1 (i. 8 equal to the amount of gold present in solution; separating by filtration the resultant metallic pre cipitate; and, optionally, recycling the barren solu tion to the upstream process, 2. A process as claimed in claim 1, wherein the stabi lized alkali metal borohydnde is subilized potassium EXAMPLE 2 borohydride. Recent studies have shown that gold can be extracted 3. A process for recovering high purity metallic gold from cyanide leach liquor by either solvent extraction from an acidic solution of thiourea, thiosulphate, thio or ion exchange techniques. (P. A. Riveros, "Studies on 10 cyanate or thiourea leach liquor containing gold values, the Solvent Extraction of Gold from Cyanide Media", / o/JfydrometaUurgy. CANMET/MSL Division Re which comprises; treating the solution with stabilized alkali metal boro port MSL 88-141 (J), (19«B), in press, and P. A. Rive hydride in an amount at least stoichiometrically ros, "Advances in Ion-Exchange and Solvent Extrac equal to the amount of gold present in the solution; tion for Gold Recovery", CANMET/MSL Divison 15 separating by filtration the rcsultiint metallic precipi Report MSL 88-109 (OP&J), (1988)). Gold and associ tate; and, optionally, ated impurities can be stripped from the loaded organic recycling the barren solution to the upstream process, phase or ion exchange resin by acidified thiourea solu wherein the stabilized alkali metal borohydride is tion. On such solutions, reduction precipitation was sodium borohydride in the form of a stabilized effected by the addition of stabilized sodium borohy- 20 aqueous sodium borohydride/sodium hydroxsidc dride. The procedure involved pH adjustment to the solution. limit of precipitation, the addition of small amounts of Ven Met TM solution and filtration to separate the re 4. A process for recovering high purity metallic gold sultant precipitate. With reference to Table 4, it can be from an acidic solution of thiourea, thiosulphate, thioseen that reaction was very efficient; 90% of the gold 25 cyanate or thiourea leach liquor containing gold values. was precipitated from the strip solution based on the which comprises: addition of 1.25 moles of stabilized sodium borohydride treating the solution with stabilized alkali metal boro per mole of gold. Analysis revealed the product to have hydride in an amount at least stoichiometrically a purity of greater than 98.0%. Recovery could be equal to the amount of gold present in the solution; increased even further by the addition of excess sodium 30 separating by filtration the resultant metallic precipi borohydride. tate; and, optionally, The process for treating gold-containing eluate solu recycling the barren solution to the upstream process, tion of loaded resin was treated in a similar manner. wherein the stabilized solution contains about 12% Table 5 shows the results. Gold was substantially com by weight of sodium borohydride and about 40% pletely recovered; the barren solution contained less j j by weight of sodium hydroxide. than 2 mg/L gold. Furthermore, product analysis re 5. A process as claimed in claim 1,3 or 4, wherein the vealed the precipitate to be pure. acidic solution is a leach solution obtained in the final TABLE 4 recovery step of the acidified thiourea leaching of gold Reduction of gold from 0 5 M thiourea ore or concentrate in the presence of carbon followed stnp solution by SBH *0 by elution of the carbon by thiosulfate or thiocyanate. V - 25 mL, iniliaJ pH - 0 2 adjusted with NaOH to pH = 0.8, 6. A process as claimed in claim 1, 3 or 4, wherein the time * 5 nun al room temperature, mL of VenMel к 0.1 tnL acidic soluuon is obtained as a result of the acidification fSBH/Au = US) of gold cyanide leach liquor by acidified thiourea which Au Zn Fe CL N1 incorporates a cyanide gas recovery means. 19« 960 72 2 20 15 20 25 30 35 40 45 50 55 60 65 10 18. A process as claimed in claim 13, wherein the acidic solution is obtained as a result ofthe acidification of gold cyanide leach liquor by acidified thiourea which incorporates a cyanide gas recovery means. 19. A process as claimed in claim 13, wherein the acidic solution is obtained in the final recovery step of either the extraction of cyanide leach liquor by an or ganic solvent followed by stripping ofthe organic phase by acidified thiourea or the treatment of cyanide leach "Ч"^^ . * i * ion-exchange resin followed by elution of the resin by acidified thiourea or thiocyanate. 20. A process as claimed in claim 13, wherein the treatment of the acidic solution with alkali metal boro hydride is effected at ambient temperature and pressure. 21. A process as claimed in claim 13, wherein the acidic solution contains gold in a concentration range of 2 mg/L to about 2 g/L. 22. A process as claimed in claim 13, wherem the acidic solution is a thiourea or thiocyanate solution and has a pH in a range of 1.5 to 3.0. 23. A process as claimed in claim 13, wherein the acidic solution is a thiosulfate solution and has a pH of about 6.0. 24. A process as claimed in claim 13, wherein the acidic solution also contains silver values and/or any zinc, iron, cobalt, nickel or copper ions, • « * • *
ДивитисяДодаткова інформація
Назва патенту англійськоюDevice for extraction of gold from industrial waste
Автори англійськоюPertsov Mykola Valeriiovych
Назва патенту російськоюУстройство для извлечения золота из промышленных отходов
Автори російськоюПерцов Николай Валерьевич
МПК / Мітки
МПК: C22B 11/00, B01D 21/00
Мітки: промислових, витягнення, пристрій, золота, відходів
Код посилання
<a href="https://ua.patents.su/10-25389-pristrijj-dlya-vityagnennya-zolota-z-promislovikh-vidkhodiv.html" target="_blank" rel="follow" title="База патентів України">Пристрій для витягнення золота з промислових відходів</a>
Попередній патент: Діодний мікроскоп для неруйнівного аналізу
Наступний патент: Спосіб контролю ізоляції обмотки ротора з рідинним охолодженням електричної машини
Випадковий патент: Спосіб виконання лапароскопічної холецистектомії у хворих на жовчнокам'яну хворобу з хронічним гепатитом та цирозом печінки