Фізіологічно активний пег-кон’югат a-інтерферону, спосіб його одержання, фармацевтична композиція на його основі та спосіб лікування або профілактики імуномодуляторних захворювань
Номер патенту: 56989
Опубліковано: 16.06.2003
Формула / Реферат
1. Физиологически активный ПЭГ-конъюгат a-интерферона, имеющий формулу (I)
 ,
,
(I)
где IFNa – a-интерферон,
R и R' независимо друг от друга – низший алкил,
Х – NH или О,
n и n' – целые числа, сумма которых составляет от 600 до 1500,
и средняя молекулярная масса звеньев полиэтиленгликоля в этом конъюгате составляет от приблизительно 26000 Да до приблизительно 66000 Да.
2. Конъюгат по п. 1, в котором молекулярная масса звеньев полиэтиленгликоля составляет от приблизительно 35000 Да до приблизительно 45000 Да.
3. Конъюгат по п. 2, в котором молекулярная масса звеньев полиэтиленгликоля составляет приблизительно 40000 Да.
4. Конъюгат по п. 1, в котором R и R' – метил.
5. Конъюгат по п. 1, в котором Х – NH.
6. Конъюгат по п. 1, в котором IFNa – a2а-интерферон.
7. Конъюгат по п. 1, в котором средняя сумма n и n' составляет от 850 до 1000.
8. Конъюгат по п. 1, в котором R и R' – метил, Х – NH, IFNa – a2а-интерферон, и один из n и n' или каждый из них равен 420.
9. Конъюгат по п. 1, в котором R и R' – метил, Х – NH, IFNa – a2а-интерферон, и один из n и n' или каждый из них равен 520.
10. Конъюгат по п. 1, который обладает более высокой антипролиферативной активностью по сравнению с a-интерфероном и меньшей антивирусной активностью по сравнению с a-интерфероном.
11. Способ получения ПЭГ-конъюгата a-интерферона, обладающего большей антипролиферативной активностью и меньшей антивирусной активностью по сравнению с a-интерфероном, состоящий в ковалентном связывании реагента формулы (II)
 ,
,
(II)
где R и R' независимо друг от друга – низший алкил,
Х – NH или О,
n и n' – целые числа, сумма которых составляет от 600 до 1500,
с a-интерфероном, с получением упомянутого ПЭГ-конъюгата a-интерферона.
12. Фармацевтическая композиция для лечения или профилактики иммуномодуляторных нарушений, таких, как заболевания, относящиеся к опухоли, и инфекционных болезней, содержащая ПЭГ-конъюгат a-интерферона, по любому из пп. 1-10 и терапевтически инертный носитель.
13. Способ лечения или профилактики иммуномодуляторных заболеваний, включающий введение ПЭГ-конъюгата a-интерферона по любому из пп. 1-10.
Текст
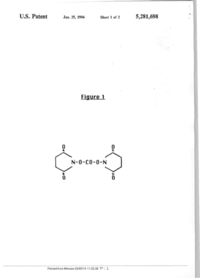
З'^оЯБЧд US005281698A United States Patent [i9] Nitecki [11] Patent Number: [45] Date of Patent: [54] 5,281,698 Jan. 25, 1994 3,235,028 8/1993 Barany ei al PREPARATION OF AN ACTIVATED POLYMER ESTER FOR PROTEIN CONJUGATION OTHER PUBLICATIONS [75] Inventor: D&nute E. Nitecki, Berkeley, Calif. [73] Cetus Oncology Corporation, Emeryville, Calif. Assignee: JuL23, IWl [51] Int. а . ї C07D 207/404; C07K 3/08; C07K 15/06; C07K 15/26 [52] U.S. a 530/351; 530/405; 530/409; 548/520; 548/545; 548/547 [58] Field of Search 530/409, 405, 351; 548/520, 545. 546, 547 [36] References Cited U.S. PATENT DOCUMENTS 4,341.707 7/1982 Ogura el al 4,904,584 2/1990 Shaw Polymer Preprints, vol. 31, No. 2, Aug. 1990, pp. 173-174 and 213-214. Primary Examiner—Jeffrey E. Russel Attorney, Agent, or Firm—Grant D. Green; Kenneth M. Goldman; Philip L. McGarrigle, Jr. [21] Appl. No.: 734,749 [22] Filed: 530/334 ABSTRACT [57] The present invention is a process for preparing an activated ester of polyethylene glycol or a polyoxyethylated polyol. After the activated ester is prepared, it can be reacted with a protem to form a polymer/protein conjugate. Conjugation with a polymer can reduce the protein's immunogenicity, increase its solubility, and increase its circulating in vivo half-iifc. Preferred proleins are IL-2, CSFs, and interferons 530/33S 514/8 20 Claims, 2 Drawing Sheets N-0-CO-O-N Printedfrom Mimosa 03/03/14 11:22.28 P . . 1 U.S. Patent Jan. 25, 1994 Sheet 1 of 2 Figure 1 0 0 u N-0-CO-O-N 0 Printedfrom Mimosa 03/03/14 11-гг^гз TV 2 n 0 5,281,698 U.S. Patent Jan. 25, 1994 Sheet 2 of 2 Figure 2 9 PEG-O-CO-O-N 0 Printedfrom Mimosa 03/03/14 11-22:29 T'*'.; 3 5,281,698 • !1,698 2 substrate protein than another derivative which also PREPARATION OF AN ACTIVATED POLYMER yields stable urethane Unkage Additionally, usmg DSC ESTER FOR PROTEIN CONJUGATION is more advantageous than prior art methods for producmg the active esler of FIG 2 These earlier methods FIELD OF THE INVENTION 5 to produce the same esler are disadyantageous because The present mvemion describes a method for prepar they use unstable leactants (chlorocarbonates of Nmg an activated polymer esler Once the activated poly hydroxysuccimide) and involve use of phosgene at the mer ester IS formed it can be used to chemically modify reaction site, which is quite disadvantageous a protein ,0 BRIEF DESCRIPTION OF THE DRAWINGS BACKGROUND OF THE INVENTION FIG 1 shows the chemical structure of DSC FIG 2 shows the chemical structiire of the activated Vanous natural and recombinant protems have medi cal and pharmaceutical utility Once they have been ester punfied, separated, and formulated, they can be parenDETAILED DESCRIPTION OF THE terally admimstered to disadvantaged hosts However, 15 INVENTION parenterally administered proteins may stimulate an immune response, may be relatively water msoluble, As mentioned above, the present invention is a pro and may have suboptimal pharmokinetic behavior cess designed to produce an activated polymer for con Consequently, It can be difficult to achieve therapeuti jugation to a protein This process can stably solubihze, cally useful blood levels m patients reduce the immunogenicity, and increase the circulating These problems may be overcome bv conjugating the half-life of proteins protems to polymers For example, polyethylene glycol Polymers (PEG) can be conjugated to proteins for various pur In a specific embodiment of the present invention, a poses Davis et al U S Pat No 4,179,337 discloses purified protein is covalently conjugated to a homopolconjugating polyethylene glycol to polypeptides, such ymer of polyethylene glycol (PEG) or a polyoxye as enzymes and msuUn Davis ei al made these conju thylated polyol (POP) PEG IS soluble m water at room gates so that the protein would be less immunogenic and temperature and has the general formula R(0—CHwould retain a substantial proportion of its physiolog 2—СН2)лО—R where R can be hydrogen, or a protecical activity Davis et al also disclose methods for plac ,Q tive group such as an aikyl or alkanol group Preferably, ing a reactive group on PEG and subsequently conju the protective group has between 1 and 8 carbons, more gating It to a protein Iwashita et al U S Pat No preferably It is methyl The symbol n is a positive inte 4,412,989, disclose covalently conjugating polyethylene ger, preferably between 1 and 1,000, more preferably glycol to an oxygen carrying molecule This conjugate between 2 and 500 The PEG has a preferred average IS useful as a blood substitute Veronese et al, Applied Biochem and Biotech, II 141-152 (1985) disclose acti 35 molecular weight between 1000 and 40,000, more pref erably between 2000 and 20,000, most preferably be vating polyethylene glycols with phenyl chlorofonnates tween 3,000 and 12,000 Preferably, PEG has at least to modify a nbonuclease and a superoxide dismutase one hydroxy group, more preferably it is a terminal Katreetal U S Pat No 4,766,106 also disclose solubi hydroxy group It IS this hydroxy group which is prefcrlizing proteins by polymer conjugation For example, PEG, and other polymers, are conjugated to recombi 40 ably activated to react with a free amino group on the protein However, It will be understood that the type nant proteins to reduce immunogenicity and increase ш and amount of the reactive groups may be varied to vivo blood levels, among other things These com achieve a covalently conjugated PEG/protein of the pounds may more specifically include interleuldn-2 present invention (lL-2), interferon-/? (IFN-/3), immunotoxms, and other proteins that share similar characteristics Nishimura et 45 Water soluble polyoxyethylated polyols are all useful al, European Patent Application 154,316 and Tomasi in the present invention They include polyoxye Interational Application Number PCT/US85/02572, thylated sorbitol, polyoxyethylated glucose, polyoxye disclose similar subject matter thylated glycerol (POG). etc POG IS preferred One reason IS because the glycerol backbone of polyoxyeThe process for attaching PEG to these useful recom bmant proteins is important Accordingly, the present 50 thylated glycerol is the same backbone occurnng natu rally m, for example, animak and humans in mono , d i , invention IS an advantageous modification in the pro triglycerides Therefore, this branching would not nec cess for preparing a PEG active ester essarily be seen as a foreign agent ш the body The POG SUMMARY OF THE INVENTION has a preferred molecular weight in the same range as The present invention is an activated polymer for 55 PEG The structure for POG is shown in Knauf et al J BiO Chem, 263 15064-15070(1988) and a discussion of attachment to a protein More specifically, the present POG/EL-2 conjugates is found in U S Pat No mvcntion IS a method for producing an activated ester 4,766,106, both of which are hereby incorporated by of polyethylene glycol (PEG) or a polyoxyethylated reference in their entireties polyol, comprising contacting a PEG or a polyoxye thylated polyol, which has at least one hydroxyl group, 60 The following discussion is directed to the conjuga with disuccimraidylcarbonate (DSC) under the appro tion of these water soluble polymers to IL-2 as a repre priate reaction conditions to form a PEG or a polyoxye sentative protein It should be understood that even thylated polyol active ester though PEG or POG is mentioned, the other recited water soluble polymers can be used Furthermore, it Among other factors, it has been discovered that the present method can produce an active PEG ester (see 65 should be understood that other proteins besides IL 2 FIG 2) that will react with proteins to yield a stable can be conjugated to the water soluble polymer For urethane linkage between the polymer and the protein example, proteins which have free amino groups can be The present activated esier reacts quicker with the conjugated Other examples of representative protems Printedfrom Mimosa 03/03/14 11 22 30 T^ 4 •k *i ' 5,281,698 include colony stimulating factors and imerferons It should be understood that the discussion of the specific proteins can also apply to other proteins as the tech niques to produce and purify the proteins can be gener ally similar 5 The PEG or POG IS attached to the protein by covalent conjugation "Covalently conjugated" or "conju gated" refer to the attachment of PEG or POG to a protein via an activated PEG or POG "Active" or "activated" describes the attachment of a reactive 10 group onto a PEG or POG hydroxyl (—OH) group, so that they can be conjugated to the protein Generally, the PEG or POG molecule is activated by attaching the reactive group to a hydroxyl group and then the active molecule is covalently conjugated to an amino group on 15 the protein While conjugation may occur between any reactive ammo acids on the protem, the reactive ammo acid IS preferably lysine The lysine is linked to a reac tive group on PEG or POG through its free e-amino group 20 Proteins As staled above, PEG or POG is attached to IL-2 as an example of a protein Interleukin-2 (IL-2) is a lymphokme which is produced by normal peripheral blood lymphocytes and is present in the body at low concen- 25 trations It induces the proliferation of antigen or mito gen stimulated T cells after exposure to plant lectins, antigens, or other stimuli lL-2 was first described by Morgan, D A , et al, Science (1976) 193 1007-1008 and originally called T cell growth factor because of its 30 ability to induce proliferation of stimulated T lympho cytes It IS a glyco-protein with a reported native molec ular weight in the approximate range of 13,000 to 17,000 daltons (S Gtllis and J Watson, J Exp Med 1980. 159 1709) and has an isoelectric point m the approxi- 35 mate range of 6-8 5 It is now recognized that in addi tion to lis growth factor properties, it modulates various Ш vitro and in VIVO functions of the immune system IL-2 IS one of several l>mphocyte produced messen ger/regulatory molecules that mediate cellular interac- 40 tions and functions With plasmed pLWl (deposited at the American Type Culture Collection on Aug 4, 1983 by Cetus Corpora tion under the provisions of the Budapest Treaty and having accession No 39,405) Synthetic recombmant IL-2 can also be made in eukaryotes, such as yeast or human cells Processes for growing, harvesting, disrupting, or extracting the IL-2 from cells are substantially de scribed m U S Pat Nos 4,604,377,4,738,927,4.656,132, 4,569,790, 4,748,234, 4.530,787, 4,572,298, 4,518,584, 4,752,585. U S Ser Nos 167,144 (now abandoned); 48,408 (now abandoned) and 200,741 (now abandoned) which are hereby incorporated by reference m their entireties Other procedures for punfying native IL-2 from T cells are descnbed by Watson, J ct al., / Exp Med. 1979, 150 849-861, Gillis, S , et al, ./. Immunol ogy, 1980, 124 1954-1962, Mochizuki, D Y, et al, / ImmunMelh. 1980,39 185-201, Welte, К e t a l . J Exp Med. 1982, 156 454-464, and European Patent Applica tions 83103582 9 (published Oct 26 1983 under No 92163) and 83400938 3(published Nov 16, 1983 under No 94317) which are also incorporated by reference in their entireties The present invention also includes the use of a col ony stirauldting factor (CSF) The term CSF is intended to include macrophage-CSF (M-CSF), granulocyteCSF (G-CSF), granulocyte/macrophage-CSF (GMCSF), and multi-CSF M-CSF IS a protein which exhibits the spectrum of activity understood in the art for M-CSF also known as CSF- 1, 1 e, when applied to the standard in vitro col ony stimulating a';sa> of Metcalf, 1970, J Cell Physiol, 76 89 as modified by Ralph ct al supra, it is capable of stimulating the formation of primarily macrophage col onies Native M-CSF IS a glycosylated dimer, dimerization IS reported to be necessary for activity as the mono mer is not active in the Metcalf or Ralph colony stimu lating assays or various other in vitrn bioactivity assays (Dds et al 1981, Blood 58 630-64], Das et al, 1982, / Biol Chem. 257 13679-13681 Stanley et al , 1977, J Biol Chem, 252 4305-4312, Halenbeck et al, 1989, Bto/Technology. 7 710-715) The term "M-CSF" refers Modifications to the primary structure itself, by dele to proteins that have M-CSF activity ш the assays de tion, addition or alteration of the ammo acids incorpo scribed above and are substantially homologous to the rated into the sequence during translation, can be made native sequence An example M-CSF sequence and a without destroying the activity of the protein Such 45 discussion of various deletion mutants is shown in U S modified protems, known as "muteins", are described m Pat No 4,847,201 another M-CSF is reported in U S и S Pat No 4.518,584, issued May 21, 1985, and U S Pat No 4,879,227 both of which these patents are Pat No 4,752,585, issued Jun 21, 1985, both are hereby hereby incorporated by reference in their entireties incorporated by reference m their entireties The pre cise chemical structure of IL-2 depends on a number of 50 M-CSF apparently occurs in numerous forms all factors, such as pH, glycosylation, denvitization, and which are included in the embodiments of the present other modifications as shown in и S Pat No 4,902,502 invention Human M-CSF CDNA clones coding for which is hereby incorporated by reference ш its en M-CSF proteins of three different lengths (a, 256 amino tirety acids, )3, 554 amino acids, and y, 438 ammo acids) have IL-2 can be produced by a prokaryotic microorgan- 55 been isolated from cells expressing the single M CSF gene (Wong et al, 1987,-Science, 235 1504-1508, Kawa ism or a cukaryodc cell that has been transformed with saki et al, 1985, Science 230 291-296 (see also U S Pat a native or modified human IL-2 DNA sequence Pref No 4,847,201), Ladner et al, 1987, Embo J erably, the IL-2 IS produced by transforming a prokar>6 2693-2698, Cerretti et al , 1988, Molecular Immunol. otic microorganism with DNA to produce a protem that possesses native human IL-2 activity It is un- 60 25 761-770) glycosylated when produced in E coli Bacteria are The other CSF molecules have some properties that preferred prokaryotic microorganisms for producing are similar to M-CSF as described above G-CSF is IL-2 and E coll is especially preferred For examples of more fully described in U S Pat No 4,810,643 and bacterial production of IL-2 see U S Pat Nos GM-CSF IS more fully described in U S Pat No 4,518,584,4,752,585, 4,738,927. and 4.564.593 which are 65 4 438,032, which are both hereby incorporated by refer all hereby incorporated by reference m their entireties ence m their entireties A typical transformed microorganism useful in the pres G-CSF and GM-CSF occur as monomers, as op ent invention isE. coh K-I2, strain MM294, transformed posed to M-CSF which IS a dimer G-CSF is know lo Printedfrom Mimosa 03/03/14 11 22 31 T= 5 5 5,281,698 6 stimulate granulocyte colony formation and IS reported 287 411-415, Yelverton et al, 1981, Nucleic Acid Reto have a molecular weight of approximately 30,000 search, 9 731-741, and Slreuh, PNAS {USA), GM-CSF IS able to shmulate granulocyte and/or mac78 2848-2852 The resulting polypeptides have been rophage colony formation and has a reported molecular punfied and tested for biological activities associate weight of approximately 22,000 Another CSF desig- 5 with partially punfied native human IFNs and found to nated multi-CSF (also known as IL-3) which has been possess similar activities Accordingly, such polypepreported to stimulate granulocyte and macrophage fori,des are potentially useful as antiviral, immunomodulamation and has a broad range of prohferative effects on lory, or antiprohferative agents other cells These CSFs are also included in the invenPolypeptides having IFN-a activity are also detion and are further descnbed in the following refer- 10 scnbed in U S Pat Nos 4,801,685 and 4,414,150, EP cnccs which are hereby incorporated by reference in NQ 32,134, Wcismann et al, 1982, UCLA Sym Мої their entireties Metcalf, 1986, Bhad 67 257-267, Clark Cell Bio 25 295-326, and Pestka, August 1983, Scienci a!, 1987, Science. 236 1229-1237, and Dexter, 1984, іфс American pp 37^3, which are hereby incorporated Nature. 309 746-747 by reference ш their entireties Naturally occurring interferons (IFNS) are species- 15 ^р^.у is a 146 ammo acid polypeptide which generspecific proteins, often glycoproiems, produced by vanдЦу ^^^^^ ^ , ( ^ Cys-Tyr-Cys and ends with GIu It also 0Щ cclU upon induction with viruses, double stranded ^^^ antraroUferative antitumor and tmmunoregulatory RNAs, other polynucleotides, antigens and mitogens properties IFN-y is more fully descnbed in Gray et al, Interferons exhibit multiple biological activities such as jgg^^ ^^^^^^_ ^95 503 U S Pat No 4,835,256, and EP antiviral antiproliferative immunomodulatory, and 20 ^^^ ,^7553 ,95^03 137,691, 159,714, and 136,694 anticellular functions At least three distinct types of Pyocessesfor preparing and/or punfying these protehuman interferons have been identified and character^^^ ^^^ ^^^^^ ,n the folbwmg references IFN 7, Euroized m terms of their anti viral anti-growth and activaв .„ . л t „...^ кт,, n n o n члоот \i(,f.in .„ e . tin II/чгг\ -Ti _ pean Patent Application Nos 170,917,169,907,13о,о20, t o n of natural killer cell (NK) activities They are pro^^^ ^^ p^^j. д ,.^з^,^„ ^ o WO 85/05619, IFNduced by leukocytes, lymphocytes, fibroblasts ^ d the 25 ^ ^ ^ ^^ ^^ ^^^ ^^^ j^^^, Т^ТТ^^'^ҐІІ "^:'л''Ґ^ Lf"\ ^on No 32 134, IFk-^. U S Pat Nos 4.462.940. ferons, respectively These are reported to be d fferent . ,^_ _„_ , ,(,„ ' . j ' _.- . , . _ n\ ! proteins coded for by distinct structural genes ^'530,787, 4,588,585 and *Д37,462 or European Patent Native human /3-interferon (HuIFN-/3) is generally Application No 83,069 All of the above descnbed produced by supermducmg human fibroblast cuUures 30 ^-^f^rences are hereby incorporated in their entireties with poly-IC (poly-nboinosmic acid and polynbocy^'^^' proteins that can be conjugated to polymers tidyhc acid) and isolating and punfymg the HuIFN-/? are descnbed as follows Production of TNF is shown thus produced by chromatographic and dcctrophoretic '" ^ S Pat Nos 4,677,063 and 4,677,064 Lymphotoxm techmques Protems or polypeptides which exhibit na'^ '"«^'^ ^""У descnbed in Paul et al , 1988, Ann Rev tive jS-interferon properties may also be produced using 35 ^"^'^ 6 407-438 IL-1 production is more fully derecombmant DNA technology by extracting poly-A^'^•"'b^d m Durum et al, 1985, Ann Rev Micro, nch 12s messenger RNA from vitallv induced human ^ 263-287 IL-6 production is more fully descnbed in cells, synthesizing double-stranded cDNA using the Kishimoto. \П9, Blood, 74 I-IO, and Revel, 1989, ЯхmDNA as a template, introducing the cDNA into an penentia 45 549-557 IL-4 production is more fully appropriate cloning vector, transfonning suitable mi 40 described m Paul et al, 1988, Ann Rev Micro, croorgamsms with the vector, harvestmg the bacteria 6 429-459 Kishimoto et al, 1988, ^л« Rev Micro, and extracting the HIFN-^ therefrom Nagola el al 6 485-512 also describe several of the above protems 1980. Nature 284 316 Goeddel et al, 1980, Nature The Active Ester 287 41 1. Yelverton et al, 1981, Лис Acid Res. 9 731, The present invention involves reacting a polymer, Streuki et al, 1981, PNAS (USA), 78 2848, European 45 such as PEG with disuccinimidylcarbonate to form a Patent Application Nos 28,033 (published May 6, PEG active ester (see FIG 2) However, it will be 1981), 321.134 (published Jul 15, 1981), and 34,307 obvious to one skilled ш the an that vanants of DSC (published,, Aug 26, 1981), and Belgian Patent No can be employed m the present invention For example, 837,379 (issued Jul 1, 1981) describe vanous currently di. 3, 3'sulfo-succmimidyl carbonate and other acidic used methods for the production of /^-interferon em- 50 alcohols for which chloroformate is unstable After the ploying recombinant DNA techmques The expression PEG active ester is formed, il can be reacted with a proteins or polypeptides have been punfied and tested protein to form a PEG/protein conjugate and have been found to exhibit properties similar to The PEG active ester shown in FIG 2 can be prothose of native IFNS Therefore, bacterially produced duced by methods knownin the pnor art However, the IFNs thus appear to have therapeutic use as antiviral 55 Pnor an methods that were used to produce the PEG and antitumor agents active ester are unlike the present method They ar-, Procedures for recovenng and punfying bactenally disadvantageous because they use unstable reactanis produced IFNs are described in U S Pat Nos (chlorocarbonatesofN-hydroxysuccimide) and involve 4,450,103, 4,315,852, 4,343,735, and 4,343,736 (which the use of phosgene at the reaction site, which is quite are hereby incorporated by reference in their entireties), 60 disadvantageous Dcryncketal, 1980,Л'аїиге287 193-197,andScandella Disuccinimidylcarbonate (C^HgNiOi) may be purand Komberg, 1911, Biochemistry, 10 4447 chased from such companies as Fluka Chemika-BioThe human IFN-a genes compose a multigene family chemika, etc It is also called di(N-N-succimmidyl) sharing 85-95% sequence homology (Goeddel et al, carbonate, bis-succmimidyl carbonate, or DSC, and has 1981, Л/аіиге.290 20-27, and Nagataetal, 1981,/ Imer- 65 a molecular weight of about 256 Its chemical structure feron Research. 1333-336) Several of the IFN-a genes is shown in FIG 1 have been cloned and expressed m E coli (Nagata et al DSC can be made by reaction of 1 mole of phosgene 19S0, Nature. 284 316-320 Goeddel etal, 19&0, Nature, with 2 moles of N hydroxysuccininiide under the ap Pnnted from Mimosa 03/03/14 11 22 33 P 6 7 5,281, 698 propnate reaction conditions shown ш the art, it is stable upon storage To construct the active PEG ester. PEG-OH іь dis solved at room temperature in an appropriate solvent, suchasCHCa, 0ГСН2С2 Preferably, DSC is suspended 5 in CH3CN. for example, and added to the PEG solution al a 30 mole excess or less, more preferably a 20 mole excess or less. Preferably an acylation catalyst is added between 0 and 1 hours later, preferably the catalyst is pyndine or 4-dimethylammopyndine (DMAP) The 10 mixture IS allowed to mix for preferably at least 4 hours, more preferably at least 16 hours At this point, a pre cipitate may form It is removed by filtration and dis carded Filtenng devices such as Whatman glass fiber filters (GH/B) arc acceptable The resulting solution 15 contains the PEG active ester as well as unreacted PEG (if any) and catalyst and excess DSC The PEG active ester IS precipitated by adding an ether, preferably di ethyl ether. The precipitate can be washed with appropnate solvents such as ether, redissolved and re- 20 precipitated if necessary The active ester can be assayed by a standard method for N-hydroxysuccmimide esters, i e , in 100 mM Tns buffer, pH 8-0 A precisely weighed amount (generally about 50 mg) of active ester is dissolved in 100 ml of 25 Tns buffer and the solution is immediately read in a spectrophotometer at 260 X, the molar extinction coeffi cient for released N-hydroxy-succmimide anion is 8520 By precisely observing the increase of N-hydroxy-succinimide anion liberated m time, one can calculate the 30 half-life of the ester in this solution, as well as total amount of active esler per sample weighed In this man ner I found the amount of active ester to be 91-95% in the samples weighed and the half-life m Tns buffer at ambient temperature to be about 1 minute (temperature 35 was not controlled) Polymer Protein Conjugation After the PEG active ester is formed, it can be conju gated with the protein to produce PEG-0-CO-NHProtem 40 In the final product, the PEG moiety is bound to the protein by a urethane, also called a carbamate, bond This bond is relatively stable and will keep PEG conju gated to the protein with httle or no hydrolysis under physiological conditions 45 The PEG active ester can be conjugated to a protein such as IL 2 in the following manner The PEG active ester IS preferably dissolved in an aqueous solution, such as 10 mM sodium acetate, pH 5 5 The IL-2 concentra tion IS preferably between 0 5 and 10 mg/ml IL-2, more 50 preferably between 1 and 5 mg/ml The solution has a preferred pH range between 8 and 10, more preferably between 7 5 and 9 5 m a buffer which preferably com prises OlM sodium borate or 0 IM EPPS (N- (2hydroxyethyl) piperazine-N-3-propane sulfonic acid) 55 pH 8 5, (available from Sigma) Other common buffers such as phosphate and Tns can be used However, those buffers which contain unhindered amines which would react with the active ester should not be employed The PEG active ester solution is added (at room tempera- 60 ture) to the IL-2 to a molar ratio preferably between 1 and 30 moles of PEG active esters per IL-2, more pref erably between 1 and 15 moles of PEG active eslers per mole IL-2, and most preferably about 5 moles of PEG active esters per mole IL-2 However, there may be 65 situations in which more PEG molecules should be attached to the subject protein In that case, the ratio of PEG active ester to protein will need to be increased 8 Preferably the IL-2 and PEG active ester is allowed to react for between 10 minutes and 24 hours, more prefer ably between 20-40 minutes A final yield of between 1 and 3 PEGs per IL-? is preferred More preferably, the final conjugate, contains between 2 and 3 PEGs per IL-2 The conjugates which ar-e produced by this, and pnor art methods can have a wide range of PEGs per IL 2 However, a mixture of conjugates having the specific average number of PEGs per IL-2 discussed above are preferred because they can be more bioactive. If desired, these preferred PEG/IL-2 conjugates can be purified from the reaction mixture There are many purification methods that are known to those of ordi nary skill in the art such as size exclusion chromatogra phy, hydrophobic interaction chromatography, ion exchange chromatography, preparative isoelectnc fo cusing, etc These methods can also be combined, for example, size exclusion chromatography can be com bined with ion exchange Preferably, a size separation method IS used, such as size exclusion chromatography which discriminates between molecules based on their hydrodynamic radius Hydrodynamic radius is defined ач the effective molecular radius of a particle in an aque ous environment A preferable charge separation method is ion exchange chromatography which dis criminates between molecules based on differential af finity of charged ions or molecules m solution for inert immobile charged substances The size exclusion chro matography method and the ion exchange chromatog raphy method are preferably run in the appropriate buffers and under the appropriate conditions More preferably, the size exclusion chromatography column has the appropriate sieving capacitj to size PEG/IL-2 conjugates with a molecular weight range preferably between 5,000 and 1,000 000 Examples of commercial columns are Sephacryl" S-200, S-30O, and S-400 HR (high resolution), and Superose 12 More preferably, the ion exchange chromatography column can discriminate between individual species of PEG/IL-2 conjugates ranging in isoelectric point between 4 and 9, most pref erably It can discriminate between PEG/IL-2 conju gates which range between 5.5 and 7 5 in isoelectnc charge Typically, the output from these punfication meth ods, I e size exclusion chromatography or юп exchange chromatography, is a UV (A280) absorbance profile of the eluted fractions some of which contain the conju gale To determine which fractions contain the pre ferred conjugates (among other conjugates), the frac tions can be screened against various standards Pre ferred screening methods include SDS-PAGE, isoelec tric focusing, capillary zone electrophoresis bioactivity. and pharmacokinetics Once it is known which fraction contains the preferred conjugates, those fractions may be further punfied For example, the polymer/protein conjugate mixture can be contacted with the size exclu sion chromatography column the fractions collected, then run on an SDS-PAGE gel to determine which fractions contain the preferred polymer/protein conju gates (among others) Then, the tractions of interest may be contacted with the ion exchange column, the fractions collected, and analyzed by isoelectnc focusing to determine which fractions have the preferred poly mer/protein con)Ugates Before the PEG/lL-2 conju gate mixture is subjected to chromatography, it may be initially prepared by removing impurities For example, Printedfrom Mimosa 03/03/14 11 22 34 P 7 5,281,698 salts may be removed with preparatory columns, or may be dialyzed against appropnate buffers Once the PEG/protein is punfied it may be tested for bioactivity using methods known ш the art If the con jugate IS PEG/IL-2, for example, a HT-2 cell prohfera- 5 tion assay using the MTT stain is acceptable and is similar to the assay descnbed by Gilhs, et al, 1978, / Immunol. 120 2027-2032 After the PEG/protein is produced and punfied it may be mcorpocated into a pharmaceutical composition lo because it is considered therapeutically effective for human and vetennary uses, such as cancer therapy and the treatment of infectious diseases See U S Pat No 4,902,502 which is incorporated by reference m its en tirety The PEG/protein can be formulated in a non 15 toxic, inert, pharmaceutically acceptable aqueous ear ner medium, preferably at a pH ranging from 3 to 8, more preferably ranging from 6 to 8 When used for in vivo therapy, the stenle PEG/protein composition will comprise protem dissolved in an aqueous buffer having 20 an acceptable pH upon reconstitution The FEG/protein can be formulated with a number of excipients such as ammo acids, polymers, polyols, sugars, buffers, preservatives, other proteins, etc Specific examples include octylphenoxy polyethoxy ethanol compounds, 25 polyethylene glycol monostearate compounds, poly oxyelhylene sorbitan fatty acid esters, sucrose fructose, dextrose, maltose glucose, dextran, mannitol, sorbitol, inositol, galactitol, xylitol, lactose trehalose, bovine or human serum albumin, citrate acetate Rmger's and JQ Hank's solutions, saline, phosphate, cysteine, arginine, carnitine, alanine, glycine, lysine, valine leucine, poly vinylpyrrolidone, polyethylene glycol, etc Preferably this formulation is stable for at least 6 months at 4° С 10 CH2CI2 A 20 molar excess of DSC (2 5 g) was sus pended m 25 mi of CH3CN and added to the M-PEGOH solution, followed by 0 4 ml of pyridine and another 1 5 ml pyridine after 30 minutes The suspension was stirred overnight The solution did not clanfy The precipitate was removed by fdtration using a Whatman glass microfiber filter (GF/B) and discarded The mother liquor was treated with 400 ml of dry ether The white precipitate was redissolved (twice) in 40 ml of CH2CI2 and reprecipitaled twice more with 200 ml of dry ether The yield was 1 91 g of white powder (92% active ester) The corresponding synthesis using DMAP as catalyst instead of pyndine was somewhat different, since the original reaction mixture became homogeneous and excess DSC could not be simply filtered off First, treat ment of the reaction mixture with dry ether (450 ml) yielded a gummy precipitate (mostly DSC which was removed), and required additional dry ether (400 ml) to precipitate the PEG derivative The product was reprecipitated twice from CHiCli-ether solution, the yield was 1 94 g of white powder (95% active ester) The pyndme catalyzed preparation was 92% active and had a half hfe of 0 81 minutes The DMAP cata lyzed preparation was 95 % active and had a half hfe of 1 1 minutes EXAMPLE II Assay of PEO-0-CO-NHS The PEG active ester assay is conducted ш 100 mM Tns, pH 8 0, using spectrophotometer readings at 260 X, the molar extinction coeffiaent for N-hydroxysuccmimide anion is 8520 The conjugate composition can be parenterally ad э* The active ester of monomethoxy-polyethylene gly ministered to the subject by methods known in the art col material showing an average molecular weight of This composition may contain other compounds that 6321, was weighed out at 58 400 mg, dissolved mio Tns increase the effectiveness or promote the desirable qual buffer and the spectrophotometer readings taken every ities of the protein The composition must be safe for 30 seconds The results are as follows administration via the route thai is chosen, sterile and .„ effective To mamtain the sterility and to increase the Time Second Absorbance al 260л stability of the protem, the composition is lyophilized and reconstituted pnor to use I 69300 90 1 78J30 120 Preferably, the formulation is suitable for parenteral 1 B3S60 1» administration to humans 01 animals m therapeutically ., ISO 1 B7SS0 effective amounts TTiese amounts may be determined 1 90810 210 bv the in vivo efficacy data obtained after preclinical 240 1 93950 270 1 93320 testing The following test systems are relevant when iOO I 96280 PEG/IL-2 is the conjugate T-cell mutagenesis, cyto toxic T-cell induction, natural kdler cell augmentation, ,„ IFN-/3 induction, enhancement or restoration of cellu Final absorbance at 30 minutes was I 99070 From lar immunity (e g treatment of immune deficient condi this data the calculated half-life of this ester was 1 I tions), and cell mediated anti-tumor activity. minute and 95% of the polyethylene glycol molecules The present process will now be illustrated by refer were estenfied ence lo the following examples which set forth particu- ,, EXAMPLE III larly advantageous embodiments However, it should be noted that these embodiments are illustrative and are PEG/IL-2 Conjugation not to be construed as restncting the invention in any The PEG active ester was produced m a manner way. similar to Example 11, and the lL-2 was produced m a EXAMPLE I 60 manner similar to that descnbed ш PCT Patent Publica tion WO 88/08849, published Nov 17,1988 ВпеЯу,£" PEG Active Ester Preparation coliv.d.s transformed with a plasmed containing the IL-2 gene and the appropriate regulatory sequences The E Monomethyl PEG (M-PEG-OH) was reacted with coll was induced, the lL-2 was produced, and then reDSC to produce the PEG active ester The DSC was obtained from Fluka Chemica - Biochemica Company 65 covered by the appropriate separation and purification methods 3 grams ofM-PEG-OH,ha\mg an average molecular weight of 6110 (purchased from Union Carbide, West An IL-2 solution was made, which contained 100 mM Virginia), was dissolved in approximately 25 ml of EPPS buffer and 2 mg/ml IL-2 at pH 8 5 Activated Printedfrom Mimosa 03/03/14 11 22 35 P 8 698 12 PEG esters were added to the IL-2 solution m a molar 2 A method m accordance with claim 1, wherein ratio of approximately 5 1 PEG active esters per IL-2 PEG IS activated with the DSC The solution was stirred at room temperature for 30 3 A method m accordance with claim 2, wherein the minutes The molar ratio of 12 PEGs per IL-2 was designed to produce a maximum amount of a conjugate 5 molar ratio of DSC to PEG is 30 1 or less 4 A method m accordance with claim 2, wherein the having a molar ratio of 2 or 3 PEGs per IL-2 As shown molar ratio of DSC to PEG is 20 1 or less by SDS-PAGE, approximately 60% of the conjugates 5 A method m accordance with claim 2 further comhad a molar ratio of 2 or 3 PEGs per IL-2 A similar pnsing adding a catalyst selected from the group con result was obtained when borate buffer was used sisting essentially of pyridine or 4-dimethylaminopyndme EXAMPLE IV 6 A method in accordance with claim 1, wherein the SDS-PAGE was earned out on the crude PEG-IL-2 PEG or the polyoxyethylated polyol has a average products (conjugated m EPPS) using BioRad precast molecular weight between 1.000 and 40,000 12% gels HPLC sizing was performed using a Supe 7 A method in accordance with claim 1. wherein the rose 12 column (Pharmacia) in 100 mM Na2S04, 10 mM '^ PEG or the polyoxyethylated polyol has a average Na: HPO4, pH 7 0. at 0 5 ml/minute Prior to HPLC, molecular weight between 2,000 and 20,000 SDS was removed from the PEG-IL-2 sample by ion 8 A method in accordance with claim 1, wherein the exchange, using a Q-Sepharose column The results PEG or the polyoxyethylated polyol has an average showed that the PEG-IL-2 conjugates resolved into molecular weight between 3,000 and 12,000 two discrete bands on the gels which corresponded to ^^ 9 A method in accordance with claim 6, wherein the species having 2 and 3 PEGs per IL-2 polymer IS PEG 10 A method in accordance with claim 1 further ' EXAMPLE V compnsing Conjugate Purification a) contacting the PEG or the polyoxyethylated polyol active ester with a protein under the appro For a larger scale purification the conjugates can be priate reaction conditions to form a PEG or a po concentrated using an Amicon stirred cell fined with a lyoxyethylated polyol protein conjugate YM 10 membrane The conjugate concentrate is 11 A method in accordance with claim 10, wherem washed with 50 mM sodium acetate buffer at pH 5 5 to produce a final protein concentration of 35 mg/ml The 30 the PEG or the polyoxyethylated polyol active ester is contacted with the protein in the molar ratio of between conjugate concentrate can be loaded on a Sephacryl ф 1 and 30 moles active esters to 1 mole protein S 200 HR column equilibrated with a 50 mM sodium 12 A method in accordance with claim 11, wherem acetate buffer pH 5 5, and fractions collected Selected the active ester is contacted with the protein m the fractions, based on з UV absorbance profile, can be run on reducing 12 5% SDS-PAGE and appropriate pools 35 molar ratio of between 1 and 15 moles active esters to 1 mole protein can be made to select conjugates having a molar ratio of 13 A method in accordance with claim 10, wherein 2 or 3 PEGs per IL-2 Size exclusion HPLC (a Zorbax the PEG or the polyoxyethylated polyol has a average GF 250 column and a buffer containing 30 mM sodium molecular weight between 1,000 and 40,000 phosphate at pH 7 and 100 mM sodium sulfate) can be 14 -A method in accordance with claim 10, wherein used to confirm that the pools contained predominantly 40 the PEG or the polyoxyethylated polyol has a average a molar ratio of 2 and 3 PEGs per IL-2 molecular weight between 2 000 and 20,000 The present invention has been described with refer 15 A method m accordance with claim 10, wherein ence to specific embodiments However, this applica the PEG or the polyoxyethylated polyol has an average tion is intended to cover those changes and substitutions which may be made by those skilled in the art without 45 molecular weight between 3.000 and 12,000 16 A method in accordance with claim 11, wherein departing from the spirit and the scope of the appended the active ester is a PEG active ester claims 17 A method m accordance with claim 11, wherein I claim the protein IS IL-2 I A method for producing an activated ester of poly 18 A method m accordance with claim 11, wherein ethylene glycol (PEG) or a polyoxyethylated polyol, 50 the protein is CSF comprising 19 A method in accordance with claim 11, wherein a) contacting PEG or a polyoxyethylated polyol, the protein IS interferon which has at least one hydroxyl group, with disuc 20 A method in accordance with claim 17, wherein cinimidylcarbonate (DSC) under the appropriate the PEG active esler is contacted with IL-2 in the molar reaction conditions to form a PEG or a polyoxye- 55 ratio around 5 moles of PEG esters to 1 mole lL-2 * • • • * thylated polyol active ester Printed from Mimosa 03/03/14 11 22 36 T^ 9
ДивитисяДодаткова інформація
Назва патенту англійськоюPhysiologically active peg-conjugate of a-interferon, a method for preparing the same, a pharmaceutical composition based thereon and a method for treatment or prophylaxis of immunomodular diseases
Назва патенту російськоюФизиологически активный пег-коньюгат a-интерферона, способ его получения, фармацевтическая композиция на его основе и способ лечения или профилактики иммуномодуляторных заболеваний
МПК / Мітки
МПК: A61K 31/00, A61P 35/00, A61P 31/04, A61P 37/00, A61K 47/48, A61P 31/12, A61K 38/21, C07K 14/52, C07K 1/113, C07K 1/10, C07K 14/555, A61P 31/00, A61P 37/02, C07K 17/00, C07K 14/56
Мітки: активний, імуномодуляторних, пег-кон'югат, фізіологічно, одержання, фармацевтична, профілактики, захворювань, спосіб, композиція, a-інтерферону, лікування, основі
Код посилання
<a href="https://ua.patents.su/10-56989-fiziologichno-aktivnijj-peg-konyugat-a-interferonu-sposib-jjogo-oderzhannya-farmacevtichna-kompoziciya-na-jjogo-osnovi-ta-sposib-likuvannya-abo-profilaktiki-imunomodulyatornikh-zak.html" target="_blank" rel="follow" title="База патентів України">Фізіологічно активний пег-кон’югат a-інтерферону, спосіб його одержання, фармацевтична композиція на його основі та спосіб лікування або профілактики імуномодуляторних захворювань</a>